Difference between revisions of "Main Page"
From MKMCXX
| Line 6: | Line 6: | ||
* [[Keywords and settings]] | * [[Keywords and settings]] | ||
| − | == | + | == Purpose == |
MKMCXX is a software suite for constructing and performing microkinetics simulations. | MKMCXX is a software suite for constructing and performing microkinetics simulations. | ||
| + | |||
| + | == Example simulation == | ||
| + | Below, a simple example simulation is shown. The simulation is based on the following input file. In the input file, you specify the compounds, the elementary reaction steps and the boundary conditions of the reaction. The microkinetic network is automatically constructed on the basis of the elementary reaction steps and the corresponding ordinary differential equations are solved over time. The results can be found in a series of data files as well as in ready-to-use graphs. | ||
| + | |||
| + | <pre> | ||
| + | # Example input for methanation | ||
| + | # | ||
| + | # This is an example of a comment. Comments should have a # as the first character on a line. | ||
| + | # | ||
| + | ############################################## | ||
| + | # the line below tells the program to start indexing all compounds | ||
| + | &compounds | ||
| + | settings) | ||
| + | CO; 0; 1.0 | ||
| + | H2; 0; 3.0 | ||
| + | CH4; 0; 0.0 | ||
| + | H2O; 0; 0.0 | ||
| + | CO*; 1; 0.0 | ||
| + | H*; 1; 0.0 | ||
| + | C*; 1; 0.0 | ||
| + | CH*; 1; 0.0 | ||
| + | CH2*; 1; 0.0 | ||
| + | CH3*; 1; 0.0 | ||
| + | O*; 1; 0.0 | ||
| + | OH*; 1; 0.0 | ||
| + | H2O*; 1; 0.0 | ||
| + | *; 1; 1.0 | ||
| + | ################################# | ||
| + | # the line below tells the program to start indexing all reactions | ||
| + | &reactions | ||
| + | # | ||
| + | # adsorptions / desorptions - Note that adsorptions and desorption can also be done with Arhenius (AR) equations | ||
| + | # m^2 amu K sigma sticking J/mol | ||
| + | HK; {CO} + {*} => {CO*}; 1e-19; 28; 2.73; 1; 1; 120e3 | ||
| + | HK; {H2} + 2{*} => 2{H*}; 1e-19; 2; 88; 2; 1; 80e3 | ||
| + | HK; {H2O} + {*} => {H2O*}; 1e-19; 18; 2; 2; 1; 63e3 | ||
| + | # Surface reactions | ||
| + | # vf vb Eaf Eab | ||
| + | AR; {CO*} + {*} => {C*} + {O*}; 6e11; 4e12; 65e3; 90e3 | ||
| + | AR; {C*} + {H*} => {CH*} + {*}; 1e13; 2e13; 40e3; 39e3 | ||
| + | AR; {CH*} + {H*} => {CH2*} + {*}; 2e12; 2e11; 75e3; 37e3 | ||
| + | AR; {CH2*} + {H*} => {CH3*} + {*}; 2e15; 4e13; 57e3; 47e3 | ||
| + | AR; {CH3*} + {H*} => {CH4} + 2{*}; 1e15; 3e9; 130e3; 57e3 | ||
| + | AR; 2{OH*} => {H2O*} + {O*}; 5e12; 5e13; 54e3; 28e3 | ||
| + | AR; {O*} + {H*} => {OH*} + {*}; 4e14; 5e14; 100e3; 67e3 | ||
| + | AR; {OH*} + {H*} => {H2O*} + {*}; 2e15; 4e13; 92e3; 30e3 | ||
| + | ################################# | ||
| + | # the lines below tell the program which settings we want to use | ||
| + | &settings | ||
| + | TYPE = SEQUENCERUN | ||
| + | PRESSURE = 10 | ||
| + | #REAGENTS = {CO},{H2} | ||
| + | #KEYCOMPONENTS = {CH4} | ||
| + | ################################# | ||
| + | # the lines below tell the program which runs we want to perform | ||
| + | &runs | ||
| + | # Temp; Time; AbsTol; RelTol | ||
| + | 400; 1e8; 1e-12; 1e-12 | ||
| + | 450; 1e6; 1e-12; 1e-12 | ||
| + | 500; 1e4; 1e-12; 1e-12 | ||
| + | 550; 1e2; 1e-12; 1e-12 | ||
| + | 600; 1e1; 1e-12; 1e-12 | ||
| + | 650; 1e1; 1e-12; 1e-12 | ||
| + | 700; 1e1; 1e-12; 1e-12 | ||
| + | 750; 1e1; 1e-12; 1e-12 | ||
| + | 800; 1e1; 1e-12; 1e-12 | ||
| + | 850; 1e1; 1e-12; 1e-12 | ||
| + | 900; 1e1; 1e-12; 1e-12 | ||
| + | 950; 1e1; 1e-12; 1e-12 | ||
| + | 1000; 1e1; 1e-12; 1e-12 | ||
| + | 1050; 1e1; 1e-12; 1e-12 | ||
| + | 1100; 1e1; 1e-12; 1e-12 | ||
| + | 1150; 1e1; 1e-12; 1e-12 | ||
| + | 1200; 1e1; 1e-12; 1e-12 | ||
| + | 1250; 1e1; 1e-12; 1e-12 | ||
| + | 1300; 1e1; 1e-12; 1e-12 | ||
| + | 1350; 1e1; 1e-12; 1e-12 | ||
| + | 1400; 1e1; 1e-12; 1e-12 | ||
| + | </pre> | ||
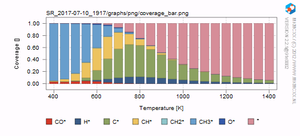
[[Image:Coverage_methane_simulation.png|thumb|Surface coverage for the methanation reaction as a function of temperature.]] | [[Image:Coverage_methane_simulation.png|thumb|Surface coverage for the methanation reaction as a function of temperature.]] | ||
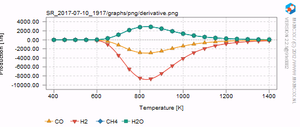
[[Image:Derivative_methane_simulation.png|thumb|Turn-over-frequency for the methanation reaction as a function of temperature.]] | [[Image:Derivative_methane_simulation.png|thumb|Turn-over-frequency for the methanation reaction as a function of temperature.]] | ||
Revision as of 17:30, 10 July 2017
The MKMCXX manual
Purpose
MKMCXX is a software suite for constructing and performing microkinetics simulations.
Example simulation
Below, a simple example simulation is shown. The simulation is based on the following input file. In the input file, you specify the compounds, the elementary reaction steps and the boundary conditions of the reaction. The microkinetic network is automatically constructed on the basis of the elementary reaction steps and the corresponding ordinary differential equations are solved over time. The results can be found in a series of data files as well as in ready-to-use graphs.
# Example input for methanation
#
# This is an example of a comment. Comments should have a # as the first character on a line.
#
##############################################
# the line below tells the program to start indexing all compounds
&compounds
settings)
CO; 0; 1.0
H2; 0; 3.0
CH4; 0; 0.0
H2O; 0; 0.0
CO*; 1; 0.0
H*; 1; 0.0
C*; 1; 0.0
CH*; 1; 0.0
CH2*; 1; 0.0
CH3*; 1; 0.0
O*; 1; 0.0
OH*; 1; 0.0
H2O*; 1; 0.0
*; 1; 1.0
#################################
# the line below tells the program to start indexing all reactions
&reactions
#
# adsorptions / desorptions - Note that adsorptions and desorption can also be done with Arhenius (AR) equations
# m^2 amu K sigma sticking J/mol
HK; {CO} + {*} => {CO*}; 1e-19; 28; 2.73; 1; 1; 120e3
HK; {H2} + 2{*} => 2{H*}; 1e-19; 2; 88; 2; 1; 80e3
HK; {H2O} + {*} => {H2O*}; 1e-19; 18; 2; 2; 1; 63e3
# Surface reactions
# vf vb Eaf Eab
AR; {CO*} + {*} => {C*} + {O*}; 6e11; 4e12; 65e3; 90e3
AR; {C*} + {H*} => {CH*} + {*}; 1e13; 2e13; 40e3; 39e3
AR; {CH*} + {H*} => {CH2*} + {*}; 2e12; 2e11; 75e3; 37e3
AR; {CH2*} + {H*} => {CH3*} + {*}; 2e15; 4e13; 57e3; 47e3
AR; {CH3*} + {H*} => {CH4} + 2{*}; 1e15; 3e9; 130e3; 57e3
AR; 2{OH*} => {H2O*} + {O*}; 5e12; 5e13; 54e3; 28e3
AR; {O*} + {H*} => {OH*} + {*}; 4e14; 5e14; 100e3; 67e3
AR; {OH*} + {H*} => {H2O*} + {*}; 2e15; 4e13; 92e3; 30e3
#################################
# the lines below tell the program which settings we want to use
&settings
TYPE = SEQUENCERUN
PRESSURE = 10
#REAGENTS = {CO},{H2}
#KEYCOMPONENTS = {CH4}
#################################
# the lines below tell the program which runs we want to perform
&runs
# Temp; Time; AbsTol; RelTol
400; 1e8; 1e-12; 1e-12
450; 1e6; 1e-12; 1e-12
500; 1e4; 1e-12; 1e-12
550; 1e2; 1e-12; 1e-12
600; 1e1; 1e-12; 1e-12
650; 1e1; 1e-12; 1e-12
700; 1e1; 1e-12; 1e-12
750; 1e1; 1e-12; 1e-12
800; 1e1; 1e-12; 1e-12
850; 1e1; 1e-12; 1e-12
900; 1e1; 1e-12; 1e-12
950; 1e1; 1e-12; 1e-12
1000; 1e1; 1e-12; 1e-12
1050; 1e1; 1e-12; 1e-12
1100; 1e1; 1e-12; 1e-12
1150; 1e1; 1e-12; 1e-12
1200; 1e1; 1e-12; 1e-12
1250; 1e1; 1e-12; 1e-12
1300; 1e1; 1e-12; 1e-12
1350; 1e1; 1e-12; 1e-12
1400; 1e1; 1e-12; 1e-12